BACKGROUND: The progress in the understanding of pathophysiology of AML has allowed the identification of genetic and immune abnormalities with prognostic impact on outcome and suitable as therapeutic targets. The genetic abnormalities are essential for risk allocation and risk-adapted treatment included the indication of hematopoietic cell transplantation. In the last decade, several studies have shown that persistence of measurable residual disease (MRD) after chemotherapy increases relapse incidence and the probability of leukemia recurrence and survival. Therefore, MRD has been progressively incorporated in prognosis estimation. In the last 25 years the CETLAM cooperative group has promoted 4 consecutive trials for AML patients fit for intensive chemotherapy and eventually HCT. Post-remission treatment was based on genetics of the disease and more recently on MRD. The aim of this study has been to investigate if the survival of patients has improved and, if so, to identify the factors that have influenced on the better outcome.
METHODS: We included all patients with primary AML up to the age of 60 years enrolled in 4 consecutive Spanish CETLAM group trials. In brief, induction chemotherapy included idarubicin, cytarabine and etoposide in AML-94, AML-99 and AML-03 protocols and without etoposide in the AML-12. G-CSF priming was allowed in the two more recent trials. Post remission therapy included 1 to 3 consolidations including intermediate or high dose cytarabine. Hematopoietic transplantation indication was based on availability of an HLA-compatible donor, genetic findings and more recently MRD. Follow-up was extended to June 2020. The survival and relapse incidence analyses were censored at 5 years. Informed consent was obtained in all cases and the institutional review boards approved the protocols.
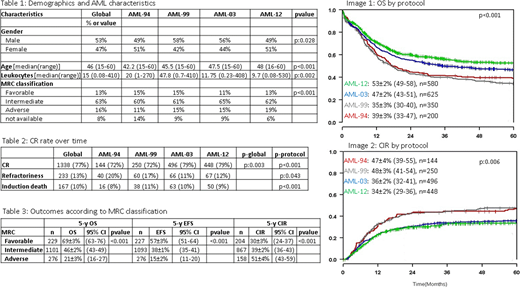
RESULTS: Between 1994 and 2019, 1755 primary AML patients between 18 and 60 years-old fulfilled the inclusion criteria. The main characteristics of patients appear in table 1. Median age of the whole group was 46 years old.
Overall survival (OS) in the whole group was 45% at 5 years, being significantly better in AML-03 and AML-12 than in AML-94 and AML-99 (image 1). Event free survival (EFS) in the whole group was 37% at 5 years, with also significant differences between trials. Also, the cumulative incidence of relapse (CIR) was 39% in the whole group with less relapses in the two more recent trials (image 2).
To understand these findings, we analyzed first the CR rate over time that was higher in the AML-03 and AML-12 protocols (table 2). The results were different depending on genetics of AML with highest CR rate in patients with CBF AML and in those with intermediate-risk cytogenetics and favorable molecular findings; in contrast, patients with adverse cytogenetics had the lowest CR rate mainly because frequent refractoriness to therapy.
According to outcomes in each MRC cytogenetic group 5y-OS was: 69±3% (63-76) in favorable group, 46±2% (43-49) in intermediate and 21±3% (16-27) in adverse group (p<0.001). 5y-EFS was 57±3% (51-64), 38±1% (35-41) and 15±2% (11-20) (p<0.001), and 5y-CIR was 30±3% (24-37), 39±2% (36-43) and 51±4% (43-59) (p<0.001), respectively (table 3).
Referent to feasibility of allogeneic HCT, there was an increased access to the procedure over the years. A higher proportion of patients allografted in AML-03 and AML-12, 32% and 41% of patients in CR, respectively, compared to 16% in AML-94 and 19% in AML-99. A shortening of the interval between CR and transplantation has been observed in recent years; 3.9 months (mo) in AML-94, 2.7 mo in AML-99, 2.9 mo in AML-03 and 2.2 mo in AML-12.
CONCLUSIONS: In adults with primary AML and age up to 60 years-old have improved over the last 25 years. During this period, the CETLAM group has refined the biological characterization of AML patients and tailored the post-remission therapy based on genetic markers with prognostic impact. The increased feasibility of allogeneic HCT may also justify the better results in more recent trials. Even though, there is substantial room for improvement, particularly in patients with AML and adverse genetic features.
Salamero:Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria; Daichii Sankyo: Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Moraleda:Takeda: Consultancy, Other: Travel Expenses; Sandoz: Consultancy, Other: Travel Expenses; Novartis: Consultancy, Other: Travel Expenses; Gilead: Consultancy, Other: Travel Expenses; Jazz Pharmaceuticals: Consultancy, Research Funding. Tormo:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; MSD: Honoraria; Daiichi Sankyo: Honoraria; Servier: Honoraria; Roche: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees. Ribera:Pfizer, Amgen: Research Funding; Pfizer, Amgen, Ariad, Novartis: Consultancy, Speakers Bureau. Sureda Balari:Roche: Honoraria; Incyte: Consultancy; Janssen: Consultancy, Honoraria; Gilead/Kite: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Merck Sharpe and Dohme: Consultancy, Honoraria, Speakers Bureau; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau; BMS: Speakers Bureau; Celgene: Consultancy, Honoraria. Sierra:Jazz Pharmaceuticals: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead-Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal